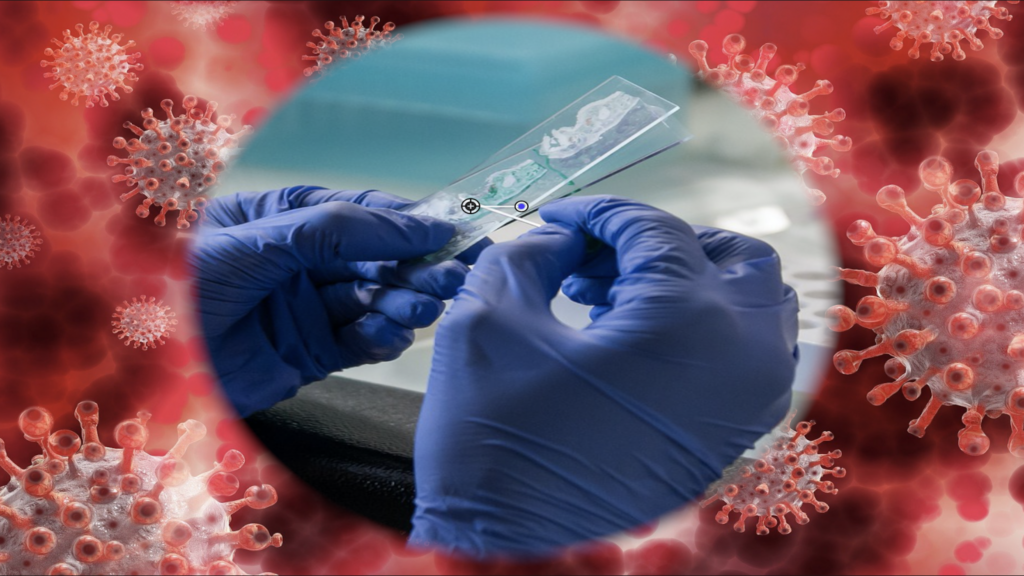
DxTerity Diagnostics announced the FDA Emergency Use Authorization (EUA 202120) for their SARS-CoV-2 RT-PCR test, SafeWorkDx™. The updated version of the saliva-based COVID-19 test can be taken unsupervised from home, as well as in the workplace.
Self-collected saliva samples are shipped for analysis and results are delivered within a short turnaround time. SafeWorkDx provides a convenient and flexible solution for at-home or on-site testing of employees, helping prevent the spread of COVID-19 while instilling peace of mind in employers and employees, alike.
DxTerity’s SARS-CoV-2 test has been authorized for testing specimens collected from individuals suspected of COVID-19 by their healthcare provider (HCP) and from any individual, including from individuals without symptoms or other reasons to suspect COVID-19.
The simple and streamlined testing process provides sample collection materials and a 48-hour stabilization kit for transport of samples, with instructions and shipping materials included.
The kit is equipped with a SDNA-1000 Saliva Collection Device and provides secure barcoded tracking of specimen to ensure the samples can be de-identified and accurately traced for security and privacy purposes.
“We have focused our efforts on adapting to the challenges of living and working during a pandemic,” says Bob Terbrueggen, Ph.D., Founder and CEO of DxTerity. “Our new at-home test provides the flexibility and reliability of results for people who are traveling, returning to work or simply want to ensure they can protect themselves and others.”
Saliva sampling is much easier to perform and suitable for unsupervised self-collection from home using the new collection kits. There is a proven concordance between saliva and nasal swab sample inputs in terms of the accuracy of results. Validation studies showed high test sensitivity, with a low Limit of Detection of 50 virus copies per milliliter of sample.

DxTerity provides customized testing solutions, with extensive experience in direct-to-patient testing. Their high-throughput DxDirect® platform enables rapid processing times and industry leading sensitivity for at home testing, supporting direct-to-patient clinical trials in autoimmune disease, COVID, and other key therapeutic areas. DxTerity will be unrolling additional ordering capabilities in the coming weeks to meet the need for increased testing during the holiday season, providing peace of mind as people return to work.
 Researchers identify antibodies that may make coronavirus vaccines unnecessary
Researchers identify antibodies that may make coronavirus vaccines unnecessary People who practice intermittent fasting experience less severe complications from COVID-19
People who practice intermittent fasting experience less severe complications from COVID-19 U.S. FDA issues risk of heart inflammation after Novavax COVID vaccine
U.S. FDA issues risk of heart inflammation after Novavax COVID vaccine Chemical found in leafy greens shown to slow growth of COVID-19 and common cold viruses
Chemical found in leafy greens shown to slow growth of COVID-19 and common cold viruses